Breakthrough Gene editing method demonstrates potential in lowering LDL Cholesterol
Ten patients, each grappling with the challenges of a genetic disorder that elevates LDL cholesterol levels to hazardous extremes, embarked on a groundbreaking experimental drug trial. Afflicted by heterozygous familial hypercholesterolemia, a condition recognized for its potential to induce severe heart disease prematurely, these individuals faced a grim prognosis. Aptly labeled as “bad cholesterol,” LDL cholesterol has long been associated with arterial blockages. Despite prior attempts with conventional cholesterol-lowering medications, the participants, considered the most critically affected, found their cholesterol levels resistant to control.
Andrew Bellinger, a distinguished cardiologist and Chief Scientific Officer at Verve Therapeutics, the Boston-based biotechnology firm spearheading the trial, noted the urgency of addressing a medical challenge that had persisted since the patients’ birth.
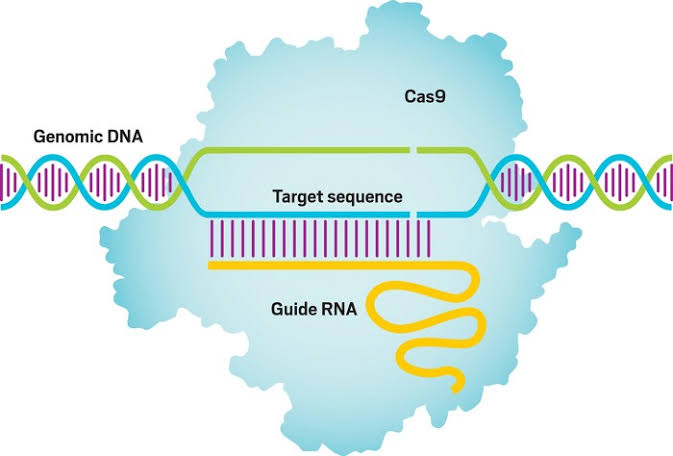
In a pioneering approach, Dr. Andrew Bellinger’s team has introduced a genetic medicine named VERVE-101, strategically crafted to deactivate a cholesterol-elevating gene. Utilizing a molecular pencil, this innovative medication precisely erases a single DNA letter and replaces it with another, effectively neutralizing the targeted gene. The revolutionary aspect lies in the potential for a lifelong impact with just a single genetic modification administered through a solitary medication. Dr. Bellinger unveiled the outcomes of the heart-1 clinical trial at the American Heart Association meeting in November, revealing that VERVE-101 successfully achieved a reduction in LDL cholesterol levels.
This groundbreaking revelation marks the first instance where an internally administered DNA spelling alteration has demonstrated a significant therapeutic effect, offering hope for a transformative approach to managing cholesterol levels with lasting implications. As Bellinger affirmed, “We can achieve clinically meaningful LDL reductions with a single dose.”
Individuals grappling with familial hypercholesterolemia, characterized by lifelong symptoms, express profound optimism about the transformative potential of a treatment like VERVE-101. Dr. Pam Taub, a cardiologist at the University of California, San Diego, not associated with the trial, underscores the groundbreaking nature of a “one and done” approach, particularly for patients accustomed to a lifetime of medication. The conventional regimen for these individuals involves continuous medication administration.
However, a groundbreaking infusion such as VERVE-101, designed to modify DNA, introduces a paradigm shift in treatment strategy. While acknowledging the potential, concerns about safety loom. Dr. Karol Watson, a cardiologist at the David Geffen School of Medicine at UCLA, emphasizes the need to rigorously establish VERVE-101’s safety, given the profound implications of editing an individual’s DNA for cholesterol management. As she aptly puts it, “You are changing the genome forever,” emphasizing the critical balance between revolutionary potential and ensuring the well-being of those undergoing such transformative interventions.

